
Laboratory of Choice
Children are not things to be molded but are people to be unfolded.
Science-first leaders whose compassion for healthcare providers and patients is incomparable.
Curabitur arcu erat, accumsan id imperdiet et, porttitor at sem. Nulla porttitor accumsan tincidunt.
Comprehensive Gastro-Intestinal Solutions
Comprehensive Gastro-Intestinal Solutions
Comprehensive Gastro-Intestinal Solutions
Anorectal Pathology with Molecular Testing
Histology
- Anal Biopsies- performed on histologic sampling for anal lesions and condyloma
- In Situ Hybridization (ISH) HPV subtyping & Immunohistochemistry (IHC) may be performed on tissue histologic samples
Cytology
Performed from ThinPrep® sample
- Anal Pap Smear- performed for cytologic screening for abnormalities
- Molecular HPV testing available with genotyping 16, 18/45
- Cytogenetic FISH testing
mRNA and Cervical Disease
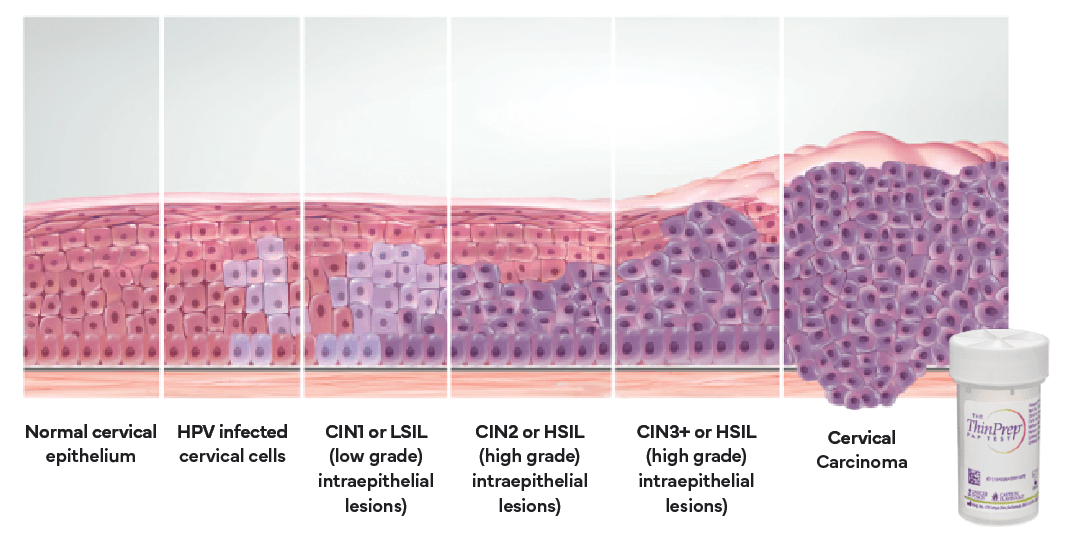
Cytogenetics
TERC FISH- A cytogenetic FISH test for identification of the amplification of the Telomerase RNA gene (TERC). The presence of this gene is likely to be associated with high grade intraepithelial lesions, most commonly linked to the Human papillomavirus, or HPV. By identifying the chromosomal instability or amplification of the gene, we are able to identify patients with this abnormality for better patient follow-up and care.
NORMAL: TERC Negative Cell
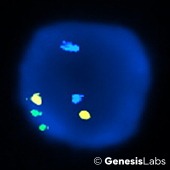
ABNORMAL: TERC Single Gain
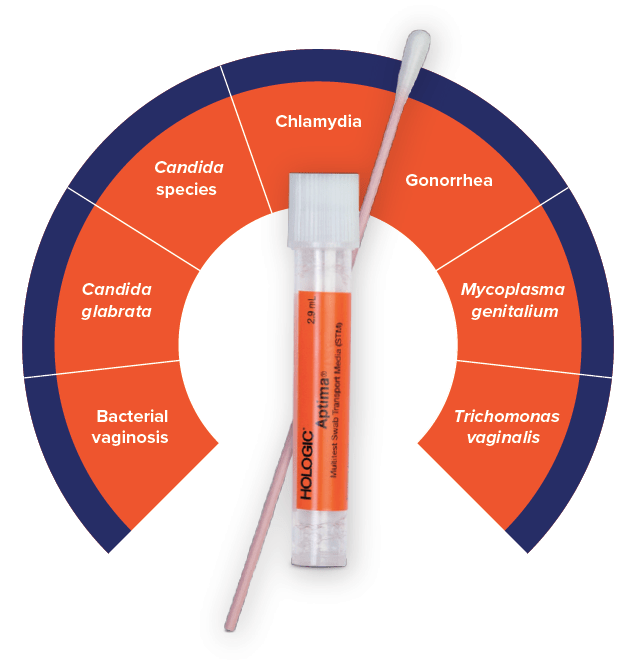
Molecular Microbiology
Performed from Aptima® Swab
- Anogenital
- Chlamydia trachomatis
- Neisseria gonorrhoeae
- Herpes Simplex Virus 1
- Herpes Simplex Virus 2
The CDC recommends chlamydia screening in the following sexually active populations:
- Women younger than 25 years old
- Women 25 years old and older with risk factors such as new or multiple sex partners or a sex partner with an STI
- All pregnant women
- Annual screening for gay, bisexual and other men who have sex with men (MSM)
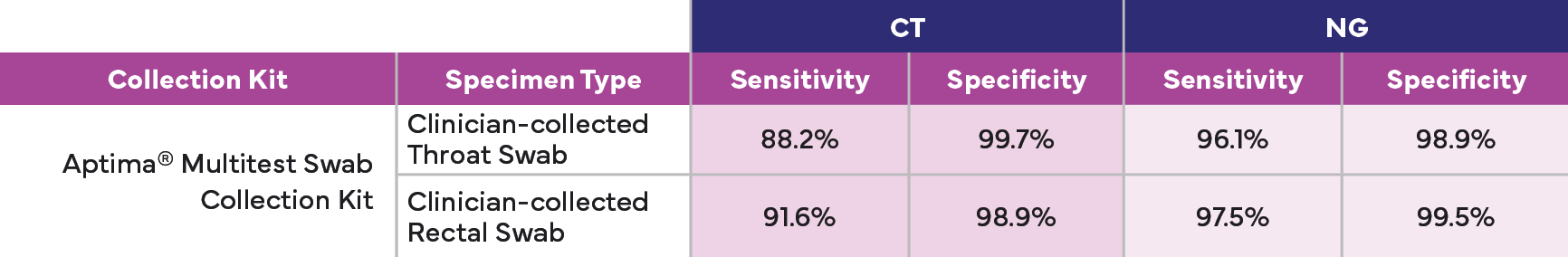
Aptima® Multitest Swab
- Clinician-collected sample: vaginal, throat, and rectal swab
- Patient-collected sample: vaginal swab
Now Available with Throat and Rectal Sample
Aptima® Combo 2 Assay
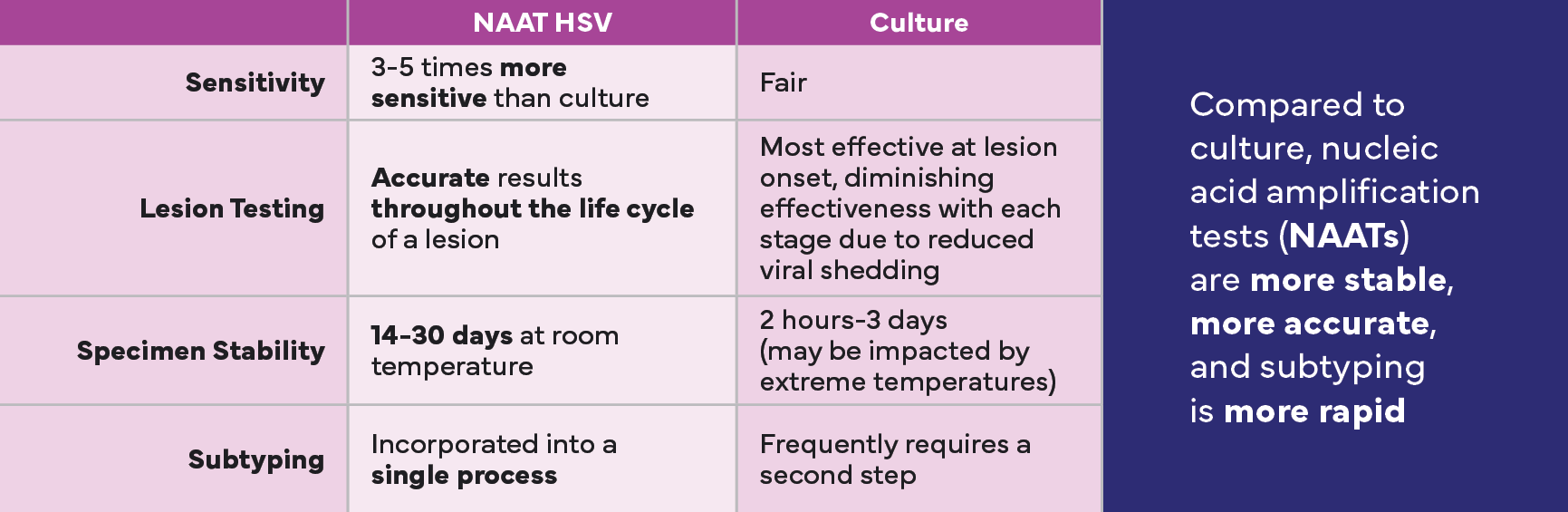
Limitations of Culture Testing Could Impact Patients
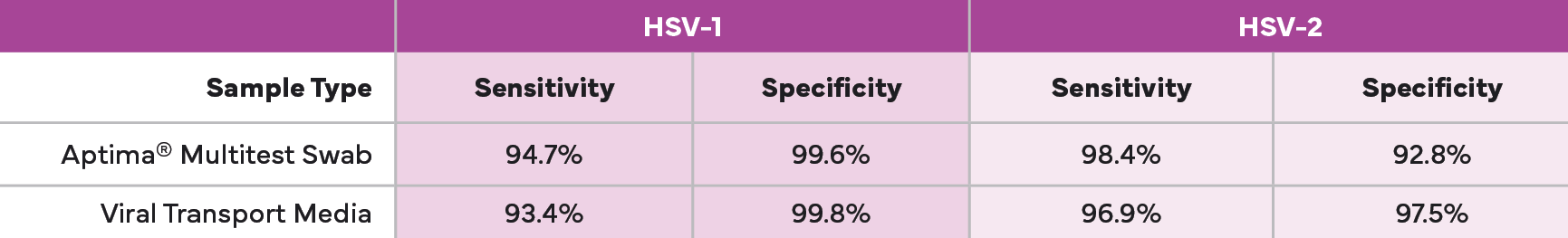
The Aptima® HSV 1 & 2 Assay: Proven Performance in Sensitivity and Specificity
Keep up-to-date with Genesis Labs.
46th Annual New York Course Conference
sean@rizco.com2022-12-02T15:58:47+00:00December 2, 2022|
Florida Digestive Diseases Update 2022
sean@rizco.com2022-12-02T16:49:20+00:00December 2, 2022|
Post ACG Conference
genesislabs222022-11-18T14:40:41+00:00November 17, 2022|
References
- CDC. Sexually Transmitted Diseases. STDs & Infertility. https://www.cdc.gov/std/Infertility/default.htm. Last reviewed October 30, 2013. Accessed May 8, 2019.
- CDC. Recommendations for the Laboratory-Based Detection of Chlamydia tachomatis and Neisseria gonorrhoeae — 2014. MMWR Recom Rep. 2014;63(RR02):1-19.
- Cernesky M, et al. High analytical sensitivity and low rates of inhibition may contribute to detection of Chlamydia trachoma’s in significantly more women by the APTIMA Combo 2 assay. J Clin Microbiol. 2006;44(2): 4:00-405. dol:10.1128/JCM.44.2.400-405.2006
- Aptima Combo 2 Assay (Panther system) [package insert]. 502466-IFU-PI. San Diego, CA; Hologic, Inc. 2018
- Chemesky M, et al. Head-to-head vaginal swabs. J Clin Microbien. 2014;52(7):2305-2310. dol:10.1125/JCM.03552-13.
- Hologic.com, HSV1 & 2 Physician Brochure, 2019.
- Hologic.com, Aptima HPV Brochure, 2018.


