
Laboratory of Choice
Children are not things to be molded but are people to be unfolded.
Science-first leaders whose compassion for healthcare providers and patients is incomparable.
Curabitur arcu erat, accumsan id imperdiet et, porttitor at sem. Nulla porttitor accumsan tincidunt.
Cytology
Urology
Standard Diagnostic Procedures
Urinary cytology screening is used for inflammatory diseases, precancerous conditions, and cancer. Cytology has a high specificity of 98% in the diagnosis of bladder cancer.
Implementation of The Paris System for Reporting Urinary Cytology was adopted and used primarily to lower atypical diagnostic rates, focus on high-grade screening and provide our clinicians with a more efficient and diagnostic way of reporting and treating a patient.
Enhanced Cytology Testing
hTERT Antibody ICC
hTERT Antibody ICC – To enhance our highly specific cytology testing, we have added an FDA-approved biomarker that aids in the detection of bladder cancer.
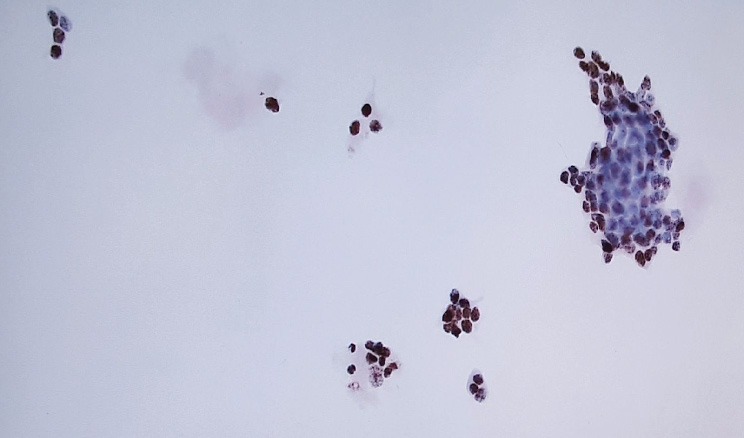
Anti -hTERT Antibody (RNA component/ telomerase reverse transcriptase)
Contains a cancer-related biomarker known as telomerase. The addition of Anti-hTERT ICC is crucial in patient care with the knowledge that 90% of bladder cancers use telomerase. When this antibody stains positive, it indicates an increased chance of urothelial cancer (including pre-invasive urothelial proliferation and invasive cancer).
Urinary STI Testing
Urine specimens may be submitted to test for sexually transmitted infections. Our testing method is through Transcription Mediated Amplification or (TMA) PCR. We currently offer STI testing to both males and females to include:
- Chlamydia trachomatis
- Neisseria gonorrhea
- Trichomonas vaginalis
- Mycoplasma genitalium
Cytology
Cytology
Keep up-to-date with Genesis Labs.
46th Annual New York Course Conference
sean@rizco.com2022-12-02T15:58:47+00:00December 2, 2022|
Florida Digestive Diseases Update 2022
sean@rizco.com2022-12-02T16:49:20+00:00December 2, 2022|


